Ficlatuzumab mechanism of action

Not actual HCP

Not actual HCP
Ficlatuzumab (anti-HGF IgG1 mAb)
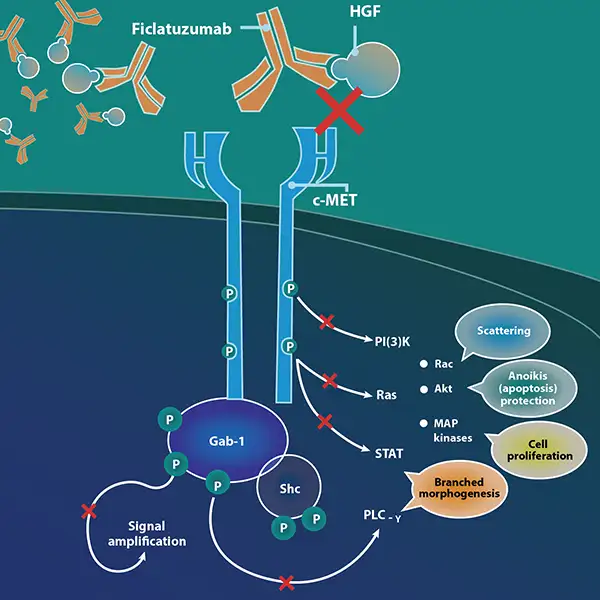
Ficlatuzumab (also known by its research code AV-299) is a humanized IgG1 monoclonal antibody that targets hepatocyte growth factor, or HGF. The HGF/cMET pathway is implicated as an escape mechanism for epidermal growth factor receptor, or EGFR, blockade. Overexpression of HGF and cMET has been reported in more than 80% of people with head and neck squamous cell carcinoma (HNSCC); EGFR overexpression is found in about 90%.
EGFR and cMET are frequently co-expressed in tumors; these pathways converge on the same downstream signaling mediators such as ERK/MAPK and PI3K/AKT. Crosstalk between EGFR and cMET has been reported in several types of cancer, making simultaneous targeting of these two pathways an important area of clinical study. Further, research has shown that acquired resistance to EGFR inhibitors tends to show amplifications in MET, which suggests that combining EGFR and cMET inhibitors upfront has potential to prevent resistance to EGFR inhibitors.
By binding to the HGF ligand with affinity and specificity, ficlatuzumab may inhibit HGF/cMET downstream signaling.

HGF/cMET as a Therapeutic Target
HGF is the sole ligand for the cMET receptor and this pathway is frequently dysregulated in a broad range of human cancers. HGF binding with cMET can activate multiple intracellular signaling pathways and lead to both tumor growth and metastatic progression of cancer cells. HGF-induced MET activation is also an escape mechanism for EGFR inhibition, leading to resistance. Ficlatuzumab has demonstrated differentiated inhibition of HGF.
- High affinity (pM) and slow off-rate for HGF
- High potency (nM) inhibiting all biological activities of HGF, including autocrine/paracrine activation loops
Ficlatuzumab in HNSCC
AVEO has reported results from a randomized Phase 2 study of ficlatuzumab as a single agent or in combination with cetuximab, an EGFR-targeted antibody, in people with R/M HNSCC who relapsed or were refractory to prior immunotherapy, chemotherapy, and cetuximab (pan-refractory), including data related to progression-free survival (PFS) and anti-tumor activity in patients who were HPV-negative randomized to the combination arm.
Based on the Phase 2 study findings, the FIERCE-HN study, a multicenter, randomized, double blind, placebo-controlled, Phase 3 clinical trial of ficlatuzumab in combination with cetuximab (vs cetuximab + placebo) in participants with HPV-negative HNSCC, has been authorized and is now enrolling participants.
FIERCE-HN study design